Disease Identification and Management in Wheat
July 25, 2021

Disease pathogens can infect wheat from seeding to ripening. Know when to look for each disease, how to identify diseases, environmental conditions that favour each disease, and options for managing wheat diseases as they become a threat throughout the growing season.
Feekes 1
Emergence/Seedling
|
Feekes 2-3
Tillering (Dormancy-Winter Wheat)
|
Feekes 4-9
Stem Elongation/Jointing
|
Feekes 10
In Boot
|
Feekes 10.1–10.5
Heading/Flowering
|
Feekes 11.1-11.2
Milk/Dough Development
|
Feekes 11.3-11.4
Ripening/Maturity
|
|---|---|---|---|---|---|---|
| Seedling blights and root rots | ||||||
| Leaf rust, Yellow rust (Stripe rust), Stem rust (typically occurs later in northern climates) | ||||||
| Wheat streak mosaic virus, Wheat spindle streak mosaic virus | ||||||
| Wheat soilborne mosaic virus | ||||||
| Barley yellow dwarf virus | ||||||
| Powdery mildew, Take-all disease, Septoria tritici blotch, Tan spot | ||||||
| Bacterial leaf blight, Bacterial leaf streak/Black chaff | ||||||
| Stagonospora nodorum blotch | ||||||
| Ergot, Loose smut, Fusarium head blight (scab) | ||||||
| Sooty head mould | ||||||
Sources
Lee, C., Herbek, J. (editors), Bruening, W., et al. 2009. A comprehensive guide to wheat management in Kentucky. ID-125. University of Kentucky Extension. http://extension.ca.uky.edu/.
Schumann, G.L. and Leonard, K.J. 2011. Stem rust of wheat. The American Phytopathological Society. https://www.apsnet.org/edcenter/disandpath/fungalbasidio/pdlessons/Pages/StemRust.aspx.
Friskop, A. and Acevedo, M. 2015. Rust diseases of wheat in North Dakota. PP1361. North Dakota State University Extension. https://www.ag.ndsu.edu/extension/.
McMullen, M. and Adhikari, T. 2009. Fungal leaf spot diseases of wheat: Tan spot, stagonospora nodorum blotch and septoria tritici blotch. PP1249. North Dakota State University Extension. https://www.ag.ndsu.edu/extension/.
Duveiller, E., Fucikovsky, L. and Rudolph, K. (editors). 1997. The bacterial diseases of wheat: concepts and methods of disease management. Mexico, D.F.: CIMMYT.
Paulsen, G.M. et al. 1997. Wheat production handbook. C-529. Kansas State University Extension. https://www.ksre.k-state.edu/agriculture/.
Burrows, M., Olmstead, J. and Grey, W. 2013. Fungal, bacterial, and physiological leaf diseases of cereal crops (wheat, durum, barley). Montana State University Extension. https://www.msuextension.org/agriculture.html.
Cowger, C. and Weisz, R. 2013. Chapter 12. Small grain disease management. Small grain production guide. North Carolina State University Extension. https://www.ces.ncsu.edu/.
Hershman, D. 2011. Black “sooty” head mold of wheat. PPFS-AG-SG-07. University of Kentucky Extension. http://extension.ca.uky.edu/.
Friskop, A., Endres, G., Hoppe, K., Mostrom, M., Ransom, J., Stokka, G. Ergot in small grains PP1904. 2018. North Dakota State University Extension. https://www.ag.ndsu.edu/publications/crops/ergot-in-small-grains.
Wheat Diseases

Figure 1. Seedling blight

Figure 2. Leaf rust. Donald Growth, Louisiana State University Ag Center, Bugwood.org.

Figure 3. Yellow (Stripe) rust. Mary Burrows, Montana State University, Bugwood.org.

Figure 4. Stem rust

Figure 5. Wheat streak mosaic virus. Mary Burrows, Montana State University, Bugwood.org

Figure 6. Wheat soilborne mosaic virus (SBWMV). H.J. Larsen, Bugwood.org

Figure 7. Barley yellow dwarf virus. Brian Olson, Oklahoma State University, Bugwood.org
)
Figure 8. Powdery mildew. Phil Sloderbeck, Kansas State University, Bugwood.org.
)
Figure 9. Take-all disease (field). William M. Brown Jr., Bugwood.org.
)
Figure 10. Take-all disease (roots). Mary Burrows, Montana State University, Bugwood.org.
)
Figure 11. Septoria tritici blotch. G.J. Holmes, Strawberry Center, Cal Poly San Luis Obispo, Bugwood.org

Figure 12. Tan spot. Mary Burrows, Montana State University, Bugwood.org

Figure 13. Bacterial leaf blight. Mary Burrows, Montana State University, Bugwood.org

Figure 14. Black chaff. Mary Burrows, Montana State University, Bugwood.org
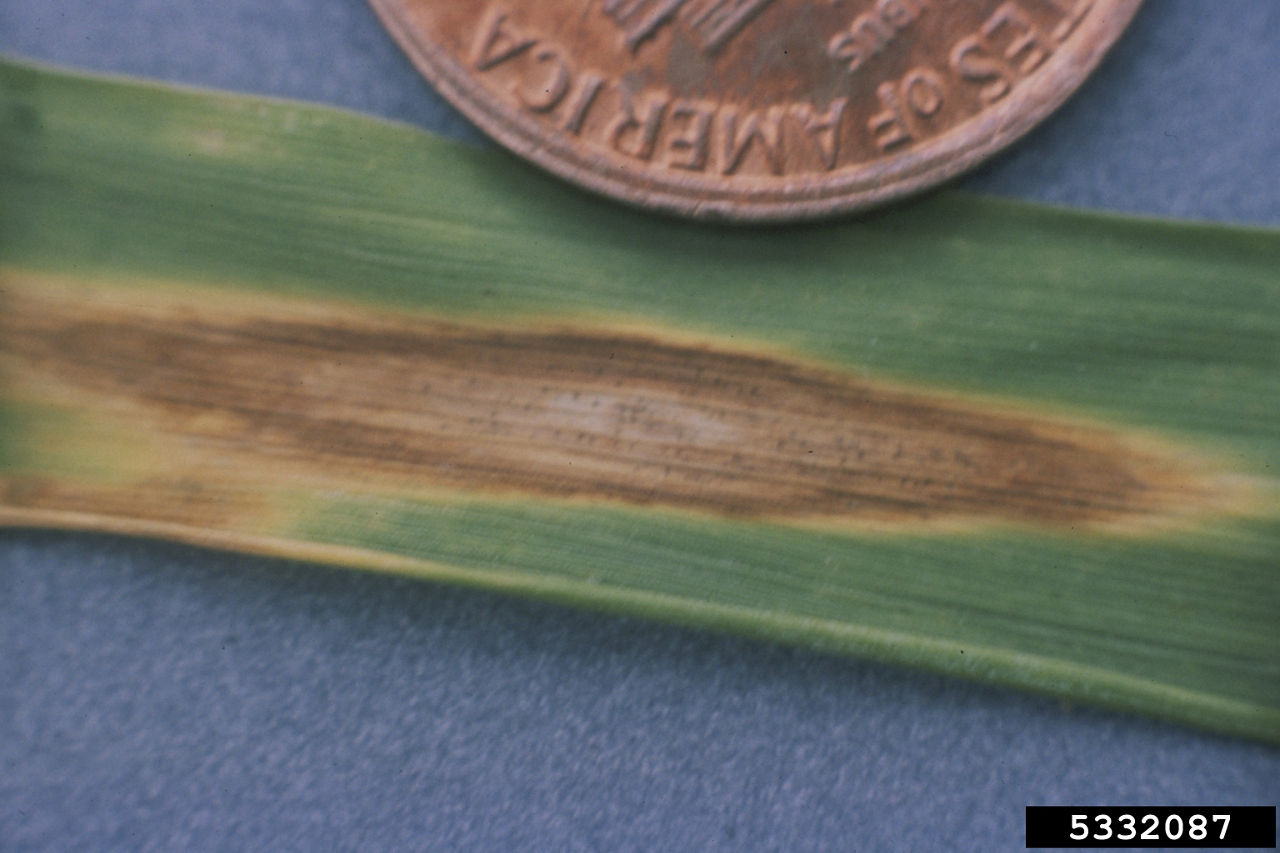
Figure 15. Stagonospora nodorum blotch (leaf). Erik Stromberg, Virginia Polytechnic Institute and State University, Bugwood.org

Figure 16. Stagonospora nodorum blotch (glume). Erik Stromberg, Virginia Polytechnic Institute and State University. Bugwood.org

Figure 17. Ergot.

Figure 18. Loose smut. Donald Groth, Louisiana State University Ag Center, Bugwood.org

Figure 19. Fusarium head blight (scab). Mary Burrows, Montana State University, Bugwood.org.

Figure 20. Sooty head mould. Mary Burrows, Montana State University, Bugwood.org.
Disease Identification, Environmental Factors, and Management
Seedling blights and root rots are a threat to wheat from emergence through the seedling stages and are caused by a complex of seed and soil-borne pathogens that can cause mortality. Cultural management practices can help encourage quick seedling growth and development with proper seed placement into warm, well-drained soil with adequate fertility. Selecting disease-free seed can help protect against seed-borne pathogens. Fungicide seed treatment registered for control of common seed and soil-borne pathogens is a chemical management option. Bayer seed treatment options include Raxil® brand seed treatments and Evergol® Energy seed treatment.
Rust diseases are fungal infections that can impact wheat from emergence through dough development.
Leaf rust (Puccinia triticina) is characterized by small, randomly distributed orange-brown lesions on upper leaf surfaces and leaf sheaths that do not coalesce. Environmental conditions that favour infection by this disease are temperatures of 15 to 20°C and at least six hours of moisture on the leaf surface. Development of the disease continues with temperatures of 20 to 25°C.
Stem rust (Puccinia graminis f.sp. tritici) pustules are larger, dark reddish-brown and can be found on upper and lower leaf surfaces, stems, and spikes. Initially the lesions will be scattered but may coalesce in heavy infestations. Environmental conditions that favour infection by this disease are temperatures of 15 to 29°C and six to eight hours of moisture on the leaf surface. Disease development continues with temperatures of 26 to 30°C.
Stripe (yellow) rust (Puccinia striiformis f. sp. tritici) is characterized by linear rows of bright yellow-orange pustules that follow leaf veins. Environmental conditions that favour infection by this disease are temperatures of 7 to 12°C and six to eight hours of moisture on the leaf surface. Development of the disease continues with temperatures of 10 to 15°C.
Rust diseases can develop rapidly in appropriate temperatures and prolonged periods of leaf wetness throughout the growing season. Severe losses can occur due to significant loss of tissue resulting in a reduction in kernels, test weight, and grain quality as well as lodging. Management includes planting resistant products where available and the use of foliar fungicides applied before an infection becomes severe. Bayer fungicides that are registered for control of leaf, stem, and stripe rust include Prosaro® XTR fungicide, Prosaro® PRO fungicide, Stratego® PRO fungicide (Eastern Canada), Delaro® fungicide (Western Canada), and TilMOR™ fungicide (Western Canada).
Wheat Streak Mosaic (WSMV)1 is vectored to winter wheat by the wheat curl mite. Spring wheat and barley can also be infected with this virus. The source of both the wheat curl mite and WSMV is volunteer wheat. Infected plants are stunted and tiller poorly. Leaves on infected plants turn yellow from the leaf tip to the leaf base, while the leaf veins usually remain green. When the mites land on a wheat plant they move to the youngest unfurling leaf and begin feeding. Loss due to WSMV depends on the variety, the weather, the percent plants infected, and the time of infection. Fall infection is the most severe and, on susceptible cultivars, can cause a loss of 80% or more. The disease is favoured by warm temperatures (greater than 21°C). Environmental stresses such as high temperatures and drought can further compound the yield loss due to WSMV as infected plants are more vulnerable to these stresses. Volunteer wheat within 0.4 to 0.8 km (¼ to ½ miles) of the new field should be killed at least two to three weeks prior to emergence. Consider planting a variety that is resistant to the virus or the curl mite. There are no chemicals effective or labeled for curl mite control.
Wheat Soil Borne Mosaic Virus (WSBMV) and Wheat Spindle Streak Mosaic Virus (WSSMV)2,3
These two viruses are both vectored by the fungus Polymyxa graminis. Infection to winter wheat can begin in the fall during periods of high soil moisture. Symptoms appear in the spring after green up but can appear in late autumn or early winter in warmer climates. In contrast to wheat streak mosaic virus, WSBMV and WSSMV diseases slow as temperatures warm. WSBMV and WSSMV can be difficult to distinguish from each other with the naked eye and can occur together on the leaves. Visual symptoms of these diseases include yellow to light green streaks on leaves, necrotic leaf tips, and plant stunting. These diseases are found in low, wet spots or areas of ample moisture. There are few management options for these diseases, but resistant cultivars may be used where available.
Barley Yellow Dwarf Virus (BYDV) is a viral disease transmitted by several species of aphid. Infection can occur in the fall or spring. However, the potential for significant symptom expression and yield loss is greatest from fall infections. Symptoms commonly occur in patches throughout the field and can include stunting, reduced tillering, and a yellow or reddish discolouration of the flag leaf, leaf tips and margins. Plants may also appear unusually erect with thickened, stiff leaves. Substantial yield loss can result. Management strategies may involve timely planting (to avoid prolonged periods of aphid feeding for viral transmission), planting resistant cultivars, insecticidal seed treatments to help reduce early-season aphid populations, and foliar insecticides to control aphid populations in the crop if thresholds are reached.
Powdery mildew4 is a fungal disease caused by Blumeria graminis f. sp. tritici. The disease is favoured by temperatures between 15 and 21°C and high relative humidity (near 100%); free moisture is not required for this disease to develop. Wheat is most susceptible to infection during stem elongation and heading. A distinctive symptom is the powdery white to gray growth on leaves, stems and heads which becomes grey-brown as the plant matures. Management includes planting resistant cultivars, balancing nitrogen fertility, managing over-wintering spores with crop rotation, residue management, and controlling volunteer wheat, and the use of fungicides. Bayer fungicides that are registered for control of powdery mildew include Prosaro® XTR fungicide, Prosaro® PRO fungicide, Stratego® PRO fungicide (Eastern Canada), Delaro® fungicide (Western Canada), and TilMOR™ fungicide (Western Canada).
Take-all5 is the root, crown and basal stem rot caused by the soil-borne fungus Gaeumannomyces graminis var. tritici. This disease is favoured by cool weather temperatures of 12 to 18°C. It is more common in neutral to alkaline soils with poor nitrogen and phosphorous fertility and poor drainage. Symptoms can appear in fall or early spring but are most commonly observed during heading. Symptoms include reduced tillering, lack of heading, bleached or empty heads, shriveled kernels, bleached plants dying prematurely, rotting roots, crown, basal culm tissue with shiny, dark-brown to black surface mat of fungal tissue beneath the leaf sheath at the base of the plants. Diseased plants pull up easily due to the rotting roots. Management includes at least a one-year break from small-seeded cereal crops with control of grassy weed hosts, adequate fertility, and delayed fall planting.
Septoria tritici blotch6 is caused by the fungal pathogen Zymoseptoria tritici. This disease is favoured by cool, wet weather (10 to 20°C and 24 hours of prolonged wetness). This disease first infects lower leaves and can impact wheat from stem elongation through ripening. Symptoms are chlorotic (yellow) flecks growing into irregular brown lesions within leaf veins. Gray to brown pycnidia pepper the lesions at later stages. Management strategies include planting resistant cultivars, using pathogen-free seed and/or fungicide seed treatment, crop rotation to reduce inoculum of pathogens in future wheat crops, and early and/or late season fungicides. Bayer fungicides that are registered for control of Septoria tritici blotch include Prosaro® XTR fungicide, Prosaro® PRO fungicide, Stratego® PRO fungicide (Eastern Canada), Delaro® fungicide (Western Canada), and TilMOR™ fungicide (Western Canada).
Septoria/Stagonospora nodorum blotch6 is caused by the fungal pathogen Parastagonospora nodorum. This disease is favoured by warm, wet weather (20 to 27°C and 12 to18 hours of wetness). Symptoms are very similar to Septoria tritici blotch though this disease presents later in the season from heading through dough development. Small chlorotic (yellow) lesions develop on lower leaves becoming red brown with a gray-brown center. Pycnidia appear in the lesions and differentiate this disease from tan spot. This disease can also infect glumes as “glume blotch,” where severe infection can result in lightweight, shriveled kernels. Management strategies include planting resistant cultivars, using pathogen-free seed and/or fungicide seed treatment, crop rotation to reduce inoculum of pathogens in future wheat crops, and early and/or late season fungicides. Bayer fungicides that are registered for control of Stagonospora nodorum blotch include Prosaro® XTR fungicide, Prosaro® PRO fungicide, and TilMOR™ fungicide (Western Canada).
Tan Spot6 is caused by fungal pathogen Pyrenophora tritici-repentis. Residue-borne spores are dispersed by wind. Infection can occur in a wide range of temperatures and is best spread during wet periods of 24 hours or more. As such, it can impact wheat through most of the season from stem elongation to ripening. Symptoms first appear as small (0.3 to 1.3 cm long by to 0.14 to 0.15 cm wide) oval to diamond-shaped spots on the leaves. These spots grow larger and become tan spots. Pycnidia is not a symptom of this disease though these spots may be found with a dark brown spot in the middle. Commonly, a yellow halo occurs around the tan spot. Kernels can also be infected developing a reddish seed coat. Management strategies include planting resistant cultivars, using pathogen-free seed and/or fungicide seed treatment, crop rotation to reduce inoculum of pathogens in future wheat crops, and early and/or late season fungicides. Bayer fungicides that are registered for control of tan spot include Prosaro® XTR fungicide, Prosaro® PRO fungicide, Stratego® PRO fungicide (Eastern Canada), Delaro® fungicide (Western Canada), and TilMOR™ fungicide (Western Canada).
Similar symptoms: Bacterial leaf blight. Bacterial leaf blight begins as small water-soaked spots, expanding and joining to form large blotches. The water-soaked appearance on or around the lesions identifies the bacterial infection which is not controlled with fungicides.7
Bacterial leaf streak, black chaff8 is a bacterial disease caused by Xanthomonas translucens pv. undulosa. Weather conditions that favour the spread of this disease are frequent storms with high winds, especially those occurring during and after flag leaf. Temperatures above 25°C favour the growth of the bacteria and symptoms appear from boot stage through dough development.9 Irrigation can also increase the risk of infection due to splashing. This disease is seed and residue-borne and is spread by splashing rain. Symptoms appear on leaves as irregular translucent water-soaked streaks turning yellow and then brown. Ooze exuded from the lesions is a key symptom of bacterial infection which can cause leaves to appear shiny when it dries. This disease can co-occur with other leaf diseases (tan spot, Septoria) making it difficult to distinguish.
Bacterial leaf streak can also infect the glumes during grain fill. This stage of the disease is referred to as “black chaff” because of the dark purple to black streaks on the glumes. A purple to yellow lesion on the stem just below the head may also appear. Severe infection can cause discolouration in kernels.
Management is limited but using clean seed may help prevent spread of the disease.
Ergot10 is a fungus that infects small grains and grassy weeds by replacing kernels with ergot bodies. Plants are susceptible to infection during flowering and wet conditions with temperatures of 15 to 26°C are most favourable for the development of this disease. The first sign of infection is a yellow drop of “honey dew” on the spike. At this stage, infection can be transmitted to other flowering plants by insects. During grain fill, the infected flower develops into a dark ergot body where there should be a kernel. Ergot infections tend to be more severe on field edges. Ergot is toxic to livestock and humans and can therefore cause downgrading of grain at the point of sale. Management includes rotating to a broadleaf crop between small grain crops, incorporating ergot bodies into the soil, managing grassy weed hosts, using ergot-free seed, and applying fungicide seed treatments and foliar fungicides. Harvesting and storing grain on field edges with high disease incidence separately may spare the rest of the grain from downgrading. Some ergot can be removed by cleaning the grain. Prosaro® PRO fungicide can be used to help suppress ergot.
Loose smut11 in wheat is a fungal disease caused by Ustilago tritici. This disease is favoured by cool (15 to 22°C), humid weather with light rain showers or heavy dew. Loose smut is seed-borne, infecting the plant systemically with symptoms first appearing at head emergence. The glumes and grain of the diseased head are replaced with dusty black powder, which is easily spread by wind, infecting flowering wheat. Loose smut contributes to yield loss as smutted heads produce no seed. Generally, this disease results in a small economic impact due to overall yield loss; however, yield losses as high as 40 percent have been reported. Management includes using resistant cultivars, using certified seed, and using a registered fungicide seed treatment such as Raxil® brand seed treatment or EverGol® Energy seed treatment.
Fusarium head blight (head scab) can become a serious problem when favourable conditions for spore production occur while wheat is blooming, and inoculum is present (commonly on corn stubble). Warm (10 to 28°C), humid weather conditions are most favourable for the development of this disease. An individual spikelet to entire heads may become infected. During grain fill, infected spikelets will turn tan to brown and may have salmon-coloured fungal growth. Grain may appear white to pinkish and shriveled with low test weight or fail to develop altogether. A crop with more than five percent infected kernels may contain enough mycotoxins to be harmful to humans and animals.12
Harvested grain containing mycotoxins may result in substantial dockage at the elevator or mill and if levels are above acceptable thresholds, the grain may be rejected. Management involves avoidance of susceptible wheat products and the use of a registered fungicide applied at early flowering when weather conditions are conducive for spore productions. Bayer fungicides registered for suppression on fusarium head blight include Prosaro® XTR fungicide, Prosaro® PRO fungicide, and TilMOR™ fungicide (Western Canada). The effectiveness of tillage and crop rotation may be limited because spores can blow in from neighbouring fields.
Sooty head mould13 is a complex of fungal diseases that grow on the heads of wheat at maturity. This disease complex is favoured by wet conditions when grain is mature or maturing. Symptoms are dark green or black mould on the surface of the wheat head. Black point is the severe case where the kernels become blackened. This disease usually does not require management in the crop year but may limit the seed quality of the crop.
Sources
1 Wolf, E.D., Webb, C.A., Zukoff, S.N. 2017. Wheat streak mosaic MF3383. Kansas State University Extension. https://www.sunflower.k-state.edu/agronomy/wheat/Wheat_Streak_Mosaic.html.
2 Freije, A., Ruhl, G., Wise, K. 2016. Wheat Viruses BP-146-W. Purdue Extension. https://www.extension.purdue.edu/.
3 Cadle-Davidson, L., Gray. S.M. 2006. Soil-borne wheat mosaic virus. Cornell University and USDA. https://www.apsnet.org/edcenter/disandpath/viral/pdlessons/Pages/SoilborneWheatMosaic.aspx.
4 Salgado, J.D., Paul, P.A. Powdery mildew of wheat. Ohio State University Extension. https://ohioline.osu.edu/factsheet/plpath-cer-11.
5 Hershman, D.E., Bachi, P.R. 2010. Take-all of wheat PPFS-AG-SG-01. University of Kentucky Extension. https://plantpathology.ca.uky.edu/.
6 Friskop, A., Liu, Z. 2016. Fungal leaf spot diseases of wheat: tan spot, Septoria/stagonospora nodorum blotch and Septoria tritici blotch. PP1249. North Dakota State University Extension. https://www.ag.ndsu.edu/publications/crops/fungal-leaf-spot-diseases-of-wheat-tan-spot-septoria-stagonospora-nodorum-blotch-and-septoria-tritici-blotch.
7 Byamukama, E. 2020. Bacterial leaf blight developing in winter wheat. South Dakota State University Extension. https://extension.sdstate.edu/bacterial-leaf-blight-developing-winter-wheat.
8 Friskop, A., Lux, L., Liu, Z. 2020. Bacterial leaf streak and black chaff of wheat PP1566. North Dakota State University Extension. https://www.ag.ndsu.edu/publications/crops/bacterial-leaf-streak-and-black-chaff-of-wheat.
9 2018. University of Minnesota Extension. https://extension.umn.edu/small-grains-pest-management/bacterial-leaf-streak-and-black-chaff-small-grains#life-cycle--1373412.
10 Friskop, A., Endres, G., Hoppe, K., Mostrom, M., Ransom, J., Stokka, G. Ergot in small grains PP1904. 2018. North Dakota State University Extension. https://www.ag.ndsu.edu/publications/crops/ergot-in-small-grains.
11 Reports on plant diseases RPD No. 112- Loose smut of wheat. 1990. http://ipm.illinois.edu/diseases/series100/rpd112/.
12 Prescott, J.M., Burnett, P.A., Saari, E.E., Ranson, J., Bowman, J., de Milliano, W., Singh, R.P., and Bekele, G. Wheat diseases and pests: a guide for field identification. CIMMYT. http://wheat.pw.usda.gov/ggpages/wheatpests.html.
13 Sooty molds and black point. Kansas State University Extension. https://www.sunflower.k-state.edu/agronomy/wheat/sooty_head_molds_black_point.html
Lee, C., Herbek, J. (editors), Bruening, W., et al. 2009. A comprehensive guide to wheat management in Kentucky. ID-125. University of Kentucky Extension. http://www.ca.uky.edu/.
Foster, J.E., Hein, G.L. 2009. Hessian fly on wheat, NebGuide G1923. University of Nebraska-Lincoln Extension. http://cropwatch.unl.edu/wheat/disease.
Hein, G.L., Kalisch, J.A., Thomas, J. 2005. Cereal aphids. NebGuide G1284. University of Nebraska-Lincoln Extension. https://cropwatch.unl.edu/wheat/disease.
Bailey, K.L., Gossen, B.D., Gugel, R.K., Morrall R.A.A. (editors). 2009. Diseases of Field Crops in Canada. The Canadian Phytopathological Society. ISBN 0-9691627-6-6.
Friskop, A., Budde-Rodriguez, S., Gill, U. 2020. Rust diseases of wheat in North Dakota PP1361. North Dakota State University Publications. https://www.ag.ndsu.edu/publications/crops/rust-diseases-of-wheat-in-north-dakota.
Wegulo, S., Byamukama, E. 2012. Rust diseases of wheat G2180. University of Nebraska-Lincoln Extension. https://extensionpubs.unl.edu/.
Web sources verified 6/17/2021.
Legal Statements
ALWAYS READ AND FOLLOW PESTICIDE LABEL DIRECTIONS. Performance may vary from location to location and from year to year, as local growing, soil and weather conditions may vary. Growers should evaluate data from multiple locations and years whenever possible and should consider the impacts of these conditions on the grower’s fields.
Tank mixtures: The applicable labeling for each product must be in the possession of the user at the time of application. Follow applicable use instructions, including application rates, precautions and restrictions of each product used in the tank mixture. Bayer has not tested all tank mix product formulations for compatibility or performance other than specifically listed by brand name. Always predetermine the compatibility of tank mixtures by mixing small proportional quantities in advance. Delaro®, EverGol®, Prosaro®, Raxil®, Stratego®, and TilMOR™ are trademarks of Bayer Group. Used under license. Bayer CropScience Inc. is a member of CropLife Canada. ©2021 Bayer Group. All rights reserved. 1039_S1_CA